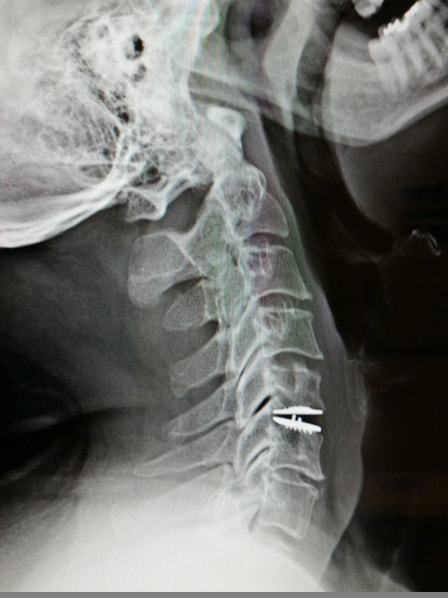
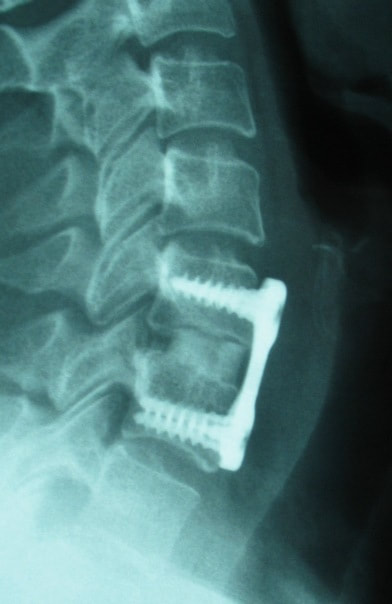
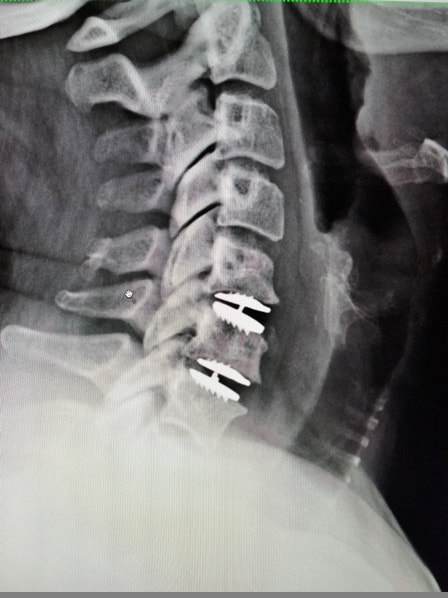
What to do after Lumbar Spine Surgery |
Details
AuthorI'm Dr. Rob McLain. I've been taking care of back and neck pain patients for more than 30 years. I'm a spine surgeon. But one of my most important jobs is... Archives
January 2024
Categories |





 RSS Feed
RSS Feed